Water
What is water? Water (H₂O) is an odorless, tasteless, and colorless liquid and substance that forms the basis of life on Earth. It is a fundamental substance without which humans, animals, and plants cannot survive. This compound is present in every living organism and can exist in three states — solid (ice), liquid, and gas (vapor). As an inorganic substance, it belongs to the realm of non-living nature.
This transparent compound consists of two hydrogen (H) atoms and one oxygen (O) atom. It can transform into ice or steam while preserving its chemical identity. Life would be impossible without it. Water covers most of Earth’s surface — approximately 71% of the planet is occupied by oceans, seas, and other bodies of water. The earliest life forms emerged in this environment millions of years ago. Thanks to its properties as a universal solvent, it enables countless chemical reactions. Chemically, water is a polar inorganic compound with the formula H₂O.
Origin of the Word
The word "water" in English originates from the Old English "wæter", which is related to the Proto-Germanic "*watōr". Its roots can be traced even further back to the Proto-Indo-European word "*wodr̥", which also gave rise to similar words in other languages, such as Latin "unda" (meaning wave or water) and Sanskrit "udán" (meaning water or rain).
The chemical formula H2O, commonly used today, was introduced after the discovery that water consists of two hydrogen atoms and one oxygen atom. This notation emphasizes the molecular composition rather than the linguistic origins of the word.
History Overview
Water's influence also extends to climate regulation and maintaining the planet's thermal balance. In many cultures, water symbolizes purity, renewal, and life itself. As a natural resource, it requires careful and responsible use. This amazing liquid has enabled our world to function as we know it since ancient times.
Water has fascinated humans for millennia, and its study evolved gradually over time.
Ancient Times
Greek philosophers, such as Thales of Miletus (c. 624–546 BCE), considered water as one of the fundamental elements and the source of life. Aristotle (384–322 BCE) included water in his system of four elements: fire, water, air, and earth.
Middle Ages
During the era of alchemy, water was studied in the context of transformations of substances. Alchemists observed boiling, condensation, and crystallization, but had no understanding of its molecular composition.
Modern Era
Joseph Priestley (1733–1804) studied the properties of gases, including their interaction with water. Antoine Lavoisier (1743–1794) demonstrated that water is composed of hydrogen and oxygen, performing experiments that showed water formation from burning hydrogen in oxygen — a key discovery in chemistry.
19th–20th Century
Scientists began investigating water as a solvent, its polarity, thermodynamic properties, and molecular structure. With advances in chemistry and physics, the study of water became essential in biochemistry, hydrology, and ecology.
The Structure of Water
Explanation: A water molecule has the chemical formula H₂O, representing its pure composition. In reality, naturally occurring water always contains dissolved substances, while distilled water — obtained through purification — is nearly free of impurities. Drinking water also contains various dissolved elements, many of which are beneficial for health.
A water molecule consists of two hydrogen (H) atoms and one oxygen (O) atom. The atoms are joined together by covalent bonds. Water can be found in rivers, lakes, swamps, seas, oceans, and underground. Under normal conditions, it is a colorless, odorless, and tasteless liquid.
In its solid state, it is called ice. Ice crystals can form snow and frost. Ice is less dense than liquid water, so it floats on water. Liquid water has its highest density at 4°C.
Water (H₂O) possesses a unique molecular structure that accounts for its many unusual and vital properties. A water molecule consists of two hydrogen (H) atoms and one oxygen (O) atom.
Molecular Geometry and Polarity
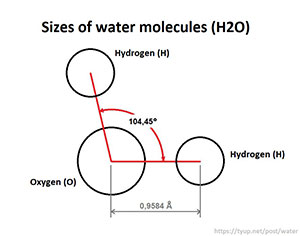
Shape: The water molecule has a bent (angular) geometry, not a straight linear one. The oxygen atom sits at the center, with the two hydrogen atoms attached at an angle of approximately 104.5∘.
Polarity: The oxygen atom is highly electronegative; it attracts the shared electrons more strongly than the hydrogen atoms do. This unequal sharing of electrons causes the electron density to shift toward the oxygen atom, giving it a partial negative charge (δ−). Conversely, the hydrogen atoms acquire a partial positive charge (δ+). This unequal distribution of charge makes the water molecule polar.
Hydrogen Bonding
The polarity of water molecules allows them to form a special type of strong intermolecular attraction known as a hydrogen bond.
- Mechanism: The partially positive hydrogen (δ+) of one water molecule is electrostatically attracted to the partially negative oxygen (δ−) of a neighboring molecule.
- Consequences: Each water molecule in the liquid state can form up to four hydrogen bonds with its neighbors. While these bonds constantly break and reform, they are strong enough to:
- Hold water molecules tightly together, contributing to water's high boiling point and surface tension.
- Force the molecules into a rigid, open tetrahedral crystalline lattice when water freezes, which is why ice is less dense than liquid water and floats.
- Make water an exceptionally effective solvent, capable of dissolving a wide range of polar and ionic substances.
Water characteristics
The gaseous form of water is steam. Water falls to the ground in the form of precipitation such as rain, snow, and hail. People use this liquid to water their crops.
Since ancient times, people have relied on rivers and lakes for survival and development. To learn more about this, read The role of water in history. There is also a general role of water for our planet.
Clean water is also important for drinking, because no living thing can survive without it. Its energy turns millstones and hydroelectric generators. Water in rivers carries people, goods, and sometimes even fallen trees.
Water is an excellent solvent. It washes away dirt. It mixes with various substances to form solutions. In natural conditions, it always contains dissolved substances. For example, salts, minerals, and gases.
Pure water does not exist in nature. It can only be obtained in a laboratory. It is called "distilled water". Distilled water does not freeze and does not conduct electricity. However, if small amounts of ionic compounds are added to such water, it becomes conductive.
Ice absorbs heat when it melts. Water releases heat when it freezes.
Seawater contains a lot of different salts, so it is not suitable for drinking.
The total volume of water on Earth is around 1400 million km³, or 1 billion 400 million km³. It covers 71% of the Earth's surface. The volume of water on Earth is not changing.
The water cycle on Earth occurs through evaporation, condensation, and precipitation. Groundwater sources make up a significant portion of the total water supply. And they are not replenished quickly.
However, the amount of fresh water on earth is decreasing. People are using it for industrial purposes. So the water is being polluted by industrial waste.
In many countries, it is prohibited to pollute water with industrial waste. Most of the earth's water (97.54%) belongs to the World Ocean.
Clean water
Fresh water is mainly found in glaciers, polar ice caps (1.81%), and groundwater (0.63%). A small part (0.009%) is collected in rivers and lakes.
Inland saltwater accounts for 0.007%. The atmosphere contains only 0.001% of all water.
The Earth's mantle contains 10-12 times more water than the World Ocean. Water expands when it changes from a liquid to a solid phase. It may contain various substances in addition to hydrogen and oxygen.
Water has tremendous force and energy. Therefore, floods, mudslides, and large waves can pose a threat to human life.
Water is one of the basic elements of life. All living organisms require clean water to survive. The water of the oceans and seas contains salts and minerals. Therefore, they are unsuitable for drinking and irrigation. Drinking seawater can be harmful to your health and can even lead to death. Agricultural plants are very sensitive to salt in water. When watered with salt water, it dries out quickly. Therefore, water from oceans and seas should not be used.
In the United Arab Emirates, Japan, and Israel, the method of purifying seawater by boiling it is used. This method separates the salt and minerals. In addition, the condensation method is used to obtain pure water from the air. This is where water is collected from the atmospheric air. For example, even in the driest Sahara, water is collected from the air.
Dew-collecting grids are used at night to collect water from the air. During the day, it is powered by solar energy or batteries. In this case, the engines are assembled by passing air through a special radiator. However, the main disadvantage of this method is its low efficiency. Therefore, in dry areas, where fresh water is scarce, it is used as a last resort.
Rainwater harvesting is considered an effective method in agricultural work. It is collected in containers through special gutters and pipes. This water is used during dry seasons through drip irrigation. At the government level, water is collected through reservoirs and ponds. This type of water can be filtered and boiled to drink. It must be boiled to kill bacteria in the water.
It is necessary to save water and protect it from pollution. Because water can quickly become scarce. Take India as an example! There, land is used for agriculture, and water is becoming scarce. Now in India (2025), they bring water from 6-9 miles away to irrigate the land. What if we also brought water from 6-9 miles away? To prevent this from happening, you need to be careful with water and conserve it.
To conserve or save water, you can use the following methods:
- Do not let water run unnecessarily;
- Use sparingly when washing;
- Taking a shower rather than a bath;
- Regularly check water pipes;
- Turning off the water when washing your face and hands;
- Water plants in the evening or morning;
- Wash fruits and vegetables in a bowl of water;
- Use of drip irrigation systems;
- Introduction of water reuse systems in industrial and commercial facilities;
- Use filters to purify dirty water;
- Use of energy-saving fittings on pipes (shower, sink, etc.);
- Operating household appliances at full load. For example, loading a washing machine with a full load of laundry without washing it first;
- Recycling of cooking water (water from various containers, without the addition of salt and other minerals), for example, for irrigation purposes;
- Rainwater harvesting, etc.
Chemical names of water
The scientific name of water is simply water, or it is denoted by the formula H₂O. However, this formula refers only to absolutely pure water, which can be obtained exclusively under laboratory conditions.
Below are several less commonly used scientific names for water.
From a formal point of view, water has several different names:
- Hydrogen monoxide: A binary compound of hydrogen and oxygen in the oxidation state of -2. (Not to be confused with hydrogen peroxide, H₂O₂.)
- Hydrogen hydroxide: A compound consisting of a hydroxyl group OH- and a cation H+.
- Hydroxyl acid: as a compound with H+ cation, which replaces water with a metal and OH- «hydroxyl residue».
- Dihydrogen monoxide.
- Dihydromonoxide.
Types of water
As mentioned at the beginning, water on Earth can exist in three main states:
- hard
- liquid
- gas
They can take different forms that interact with each other:
- water vapor and clouds in the sky;
- seawater and icebergs;
- glaciers and surface waters;
- water-bearing layers in the ground.
Types by origin, composition, and application features:
by the content of calcium cations and magnesium
- soft water
- hard water
by hydrogen isotopes in the molecule
- Light water (close in composition to regular water);
- Heavy water (deuterium);
- Very heavy water (tritium).
Other types:
- Clean water;
- Rainwater;
- Sea water (salty);
- Groundwater;
- Mineral water;
- Less salty water;
- Potable water;
- Distilled and deionized water;
- Running waters;
- Surface water;
- Pyrogen-free water;
- Bound water;
- Structured water (a term used in non-academic theories);
- Melt water (from snow, ice);
- Dead and living water are waters with mythical properties. However, the existence of such waters has not been proven.
Water that is incorporated into other substances and is physically bound to those substances is called moisture. It is classified as follows depending on the type of bond:
- Absorbed, dripped, and osmotic moisture in solids;
- Dissolved and emulsion moisture in liquids;
- Water vapor and fog in gases.
A substance that has moisture is called a moist substance. A substance that cannot absorb moisture can be called a saturated substance. Because it has too much moisture, it cannot absorb it.
In concrete usage, if a substance contains little moisture, we call it dry matter. If a hypothetical substance contains absolutely no moisture, it is called absolute dry matter. The dry matter that forms the basis of a given wet substance is the dry fraction of the wet substance.
A mixture of water vapor and gas is called wet gas (the old name was a vapor-gas mixture).
Water in nature
Main article: Water in nature
Water forms rivers, lakes, seas, and oceans, transports minerals through the soil to support plant growth, and creates atmospheric precipitation. It cleanses surfaces, shapes landscapes, and plays a key role in agriculture and irrigation.
In the Earth's atmosphere, water exists in the form of droplets, clouds, fog, and vapor. During condensation, it falls from the atmosphere in the form of precipitation. In general, the liquid water layer of the Earth is called the «hydrosphere». The solid layer is called the «cryosphere». According to theories, life on Earth originated in an aquatic environment.
Water cycle
Main article: Water cycle
The water cycle, or hydrological cycle, is the continuous movement of water through the atmosphere, land, and underground. It is essential for life, climate regulation, and shaping Earth’s environment, with water constantly changing form and location.
The complete cycle has six stages: evaporation, transpiration, condensation, precipitation, infiltration, and runoff. A simplified version has four: evaporation, condensation, precipitation, and water collection in oceans, rivers, and lakes.
Water cycle steps
- Evaporation: Water from oceans, lakes, and soil turns into vapor due to the Sun’s heat.
- Transpiration: Plants release water vapor from their leaves.
- Condensation: Water vapor cools and forms clouds, fog, or dew.
- Precipitation: Water falls as rain, snow, sleet, or hail.
- Infiltration & Percolation: Water seeps into the soil, replenishing groundwater.
- Runoff: Water flows over the land into rivers, lakes, and oceans, completing the cycle.
The importance of water
Water determines the life of all living things on Earth, including humans. It plays a unique role. Water is the source of man, the Earth, and life in general. It is a universal solvent that carries out the main biochemical processes in organisms. The uniqueness of water lies in its ability to dissolve organic and inorganic substances well. This, in turn, ensures a high speed in the course of chemical reactions, and the complex compounds formed are quite complex.
Due to hydrogen, water remains liquid over a wide temperature range. The density of ice is lower than that of liquid water. Therefore, water in ponds freezes from above, not from below. The formed layer of ice prevents the pond from freezing to the bottom. Thus, it allows life under the ice. However, there are other points of view. If water did not expand when freezing, cellular structures would not be damaged. Therefore, freezing would not harm living organisms. It is believed that newts (a type of lizard) can tolerate the freeze/thaw process. This is facilitated by the special composition of their cell plasma, which does not expand when frozen.
Growing crops in open, dry areas requires a lot of water.
The body of a living person contains 60-70% water, depending on weight and age. If the human body loses more than 12% (according to some sources up to 20%) of water, death can occur. Depending on the ambient temperature, humidity, and physical activity, a person drinks different amounts of water. There is much debate about how much water the body needs to function optimally. Therefore, it is recommended to drink water only when thirsty.
Potable water is water that has been purified from microorganisms and harmful impurities. The potability of water is determined by the number of E. coli bacteria in one liter of water. The more bacteria there are, the more resistant it is to antibacterial agents. If the number of E. coli bacteria is less than 3 per liter of water, it is considered potable. However, the potability of water is not determined by bacteria alone. The amount of minerals in water is also important. Such minerals can accumulate in the kidneys and pose a threat to human life.
In the field of sports, there are many types of sports related to water. Ice, snow, water sports and underwater sports are widespread. These include hockey, rowing, biathlon, swimming and others.
Water is used as a lubricant in bearings made of wood, plastic, and PCB. Water cutting technology is also widely used. Such a versatile substance.
According to scientific research, the need for water may increase by 55% by 2050. This raises the issue of rational use of water.
Research
The origin of water on Earth is a matter of scientific debate. Some scientists believe that it came from asteroids and comets during the early formation of the Earth (approximately 4 billion years ago). Studies conducted in 2010 determined that water in the Earth's mantle formed 2.7 billion years ago.
Hydrology is the science that studies natural water and its interactions with the atmosphere and lithosphere. It also studies the processes between the atmosphere and the lithosphere (evaporation, freezing, etc.). The subject of study of hydrology is all water in the hydrosphere. For example, oceans, seas, rivers, lakes, reservoirs, swamps, surface and groundwater.
In addition, hydrology studies other actions of water. For example, the water cycle in nature, the human impact on the cycle, the management of the regime of water bodies, the water regime of individual regions. It analyzes the hydrological elements of individual regions and the Earth as a whole. It assesses and predicts the state and rational use of water resources. It uses methods from hydrology, geography, physics and other sciences. Data from marine hydrology are used in navigation. For example, in navigation and military operations.
Hydrology is divided into three branches. They are: oceanology, terrestrial hydrology, and hydrogeology. These are further divided into the following:
- Oceanology is divided into sections such as ocean biology, chemistry, geology, physics, and ocean-atmosphere interactions.
- It is divided into land hydrology, river hydrology (potamology), lake science (limnology), marsh science, and glaciology.
- Hydrogeology is the study of the origin, location, composition, and patterns of groundwater. It also studies the interaction of groundwater with rocks, surface water, and the atmosphere. The scope of hydrogeology includes issues such as groundwater dynamics, hydrogeochemistry, water exploration and exploration, and land reclamation and regional hydrogeochemistry. Hydrogeological data are used to address issues of water supply, land reclamation, and mineral exploitation.
Interesting facts about water
Here are some of the many amazing properties of water:
- As mentioned at the beginning, pure water does not conduct electricity. The conduction of electricity occurs because of impurities in the water. Be careful, do not confuse it with drinking water. Water used in everyday life conducts electricity. Pure water, on the other hand, is obtained only in a laboratory.
- The human body dies when it loses 12% (according to some sources, up to 20%) of its water. At 10% loss, hallucinations appear. At 2% loss, thirst occurs.
- Only 2.46% of the total water on Earth is drinkable.
- Rainwater cannot be considered pure, because due to human activities, water vapor in clouds contains various impurities.
- Water can absorb and scatter light, which is why ocean water appears blue.
- Hot water cools or freezes faster than cold water. This phenomenon is called the "Mpemba effect."
- Recent studies suggest that water can exist in more than 20 different states, not just three. Under certain conditions, water can transition to a glassy, highly viscous, or elastic state.
- On average, 70% of the water on Earth used by humans is used in agriculture, and 22% in industry.
- Pure water with the chemical formula H₂O rarely occurs in nature because it usually contains dissolved substances such as salts and minerals.
- According to WHO estimates, up to 80% of diseases in developing countries are linked to unsafe drinking water. Louis Pasteur came to the same conclusion in the last century.
- As we age, the human body begins to dry out. While an embryo is 94% water, a young child is 80%, and an elderly person is 60%.
- When the body lacks water, it feels like it is hungry.
- Due to the angular shape of its molecule, water has a high surface area, which in turn allows some insects (such as water striders) to walk on water.
- Unlike most substances, water is less dense in its solid (ice) state, so ice floats in water.
- The human heart and brain are made up of 73% water.
- Water plays a key role in the body's thermal regulation, nutrient transport, and waste elimination.
- In addition to hydroelectric power plants, water is also used in geothermal and nuclear power.
- The earliest civilizations in human history (the Egyptians and Sumerians) lived near and along rivers such as the Nile, Tigris, and Euphrates, because water was essential for life and agriculture.
- Water has a high heat capacity. Therefore, it can absorb and store large amounts of heat. This property plays a key role in stabilizing the Earth's climate.
- In the religions, cultures, and philosophies of various peoples, water symbolizes life, purity, and spiritual renewal.
Protecting Our Water
Water is the most precious gift of nature and the basis of life. It is the most necessary substance for all living things on Earth. Humans, animals, and plants cannot survive without water. Therefore, water is considered the source of life.
Water makes up 70% of the human body and is involved in all life processes in our bodies. We drink water, prepare food, and maintain cleanliness. Water is also of great importance in agriculture, industry, and the energy sector. Farmers use water to grow crops, factories use water in production, and hydroelectric power plants produce electricity.
Today, the issue of clean water has become a major problem in the world. In some regions, there is a shortage of clean water, and many rivers and lakes are polluted with industrial waste. Drinking dirty water can cause various diseases. Therefore, we must use water wisely and try to keep it clean.
Using water wisely is everyone's responsibility. We should not waste it, but save it. For example, if we take steps such as not using water for unnecessary things, collecting rainwater, and distributing water properly, we can preserve our natural wealth. We should also prevent factories and industries from polluting water and harming the environment.
Water is a unique resource of nature. Keeping it clean and using it economically is our responsibility. If we use water properly and protect it, future generations will also be able to drink clean water and live healthy. Therefore, each of us must understand the value of water and take care of it!